Unlock the Secrets of Porous Materials: How Tiny Tweaks Can Create Big Changes
"Dive into the fascinating world of porous polymers and learn how manipulating emulsion techniques can revolutionize material science, one pore at a time."
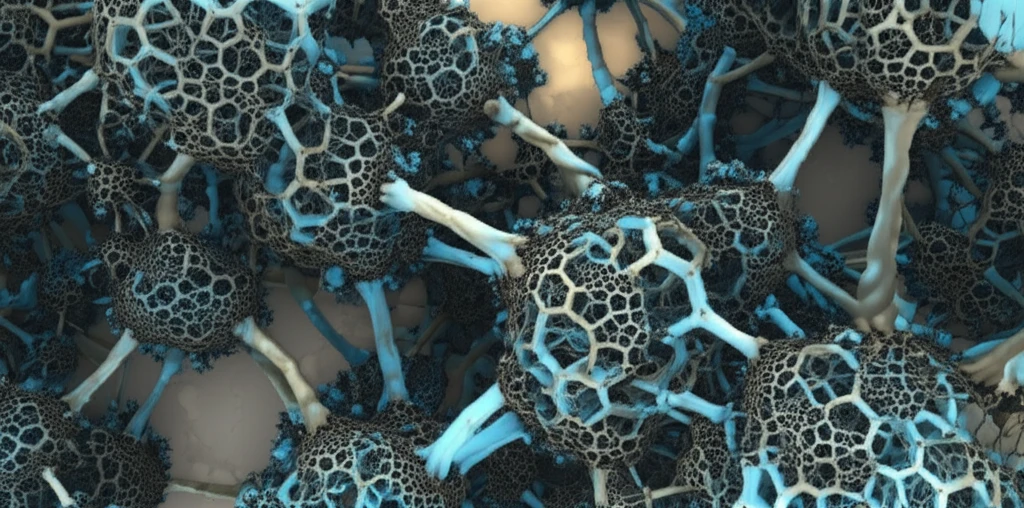
Imagine materials so full of holes they resemble a sponge at the microscopic level. These are porous materials, and they're not just fascinating to look at; they're incredibly useful. From filtering pollutants to delivering drugs, their unique structure makes them perfect for a wide range of applications.
One exciting method for creating these materials involves something called high internal phase emulsions (HIPEs). Think of it like making a salad dressing, but instead of oil and vinegar, you're mixing different liquids that don't usually combine. By carefully controlling this mixture and then solidifying it, scientists can create materials with pores of specific sizes and shapes.
Recent research has focused on creating porous poly(acrylic acid) (PAA) using HIPEs. PAA is a versatile polymer already used in diapers and absorbent materials. But by making it porous, scientists can unlock even more potential, tailoring it for specialized tasks like targeted drug delivery or advanced filtration systems.
How Does Emulsification Affect Porous Structure?

The secret to creating these custom porous materials lies in controlling the emulsification process. Emulsification is the process of dispersing one liquid into another, like when you mix oil and water with an emulsifier to create a stable mixture. In the case of porous PAA, scientists use a high internal phase emulsion, meaning one liquid makes up a large portion of the mixture.
- Type of Internal Phase: Traditionally, materials like toluene or hexane are used as the internal phase. However, using paraffin, which has a higher viscosity, can lead to more stable emulsions and allows for the use of less surfactant.
- Surfactant Concentration: Surfactants help stabilize the emulsion. By using lower concentrations of surfactants like Tween 60, the resulting material is not only more environmentally friendly but also easier to purify.
- Monomer Concentration: The amount of acrylic acid in the water phase directly affects the pore size and interconnectivity. Higher concentrations can lead to smaller, more uniform pores.
The Future of Porous Materials
The ability to tailor the structure of porous materials opens up a world of possibilities. Imagine custom-designed filters for water purification, drug delivery systems that target specific cells, or even new types of cosmetic products with enhanced absorption. As research in this area continues, we can expect to see even more innovative applications emerge, transforming industries and improving lives.
