Myeloid Checkpoint: The Future of Cancer Treatment?
"A new study reveals how blocking the CD47-SIRPa interaction can boost the effectiveness of existing cancer therapies and pave the way for innovative treatments."
The field of immuno-oncology is rapidly evolving, with researchers constantly seeking new ways to harness the power of the immune system to fight cancer. Immune-checkpoint inhibitors, which activate adaptive immunity, have become a cornerstone of cancer therapy. Now, scientists are exploring the potential of targeting innate immune checkpoints to further enhance the immune response against malignancies.
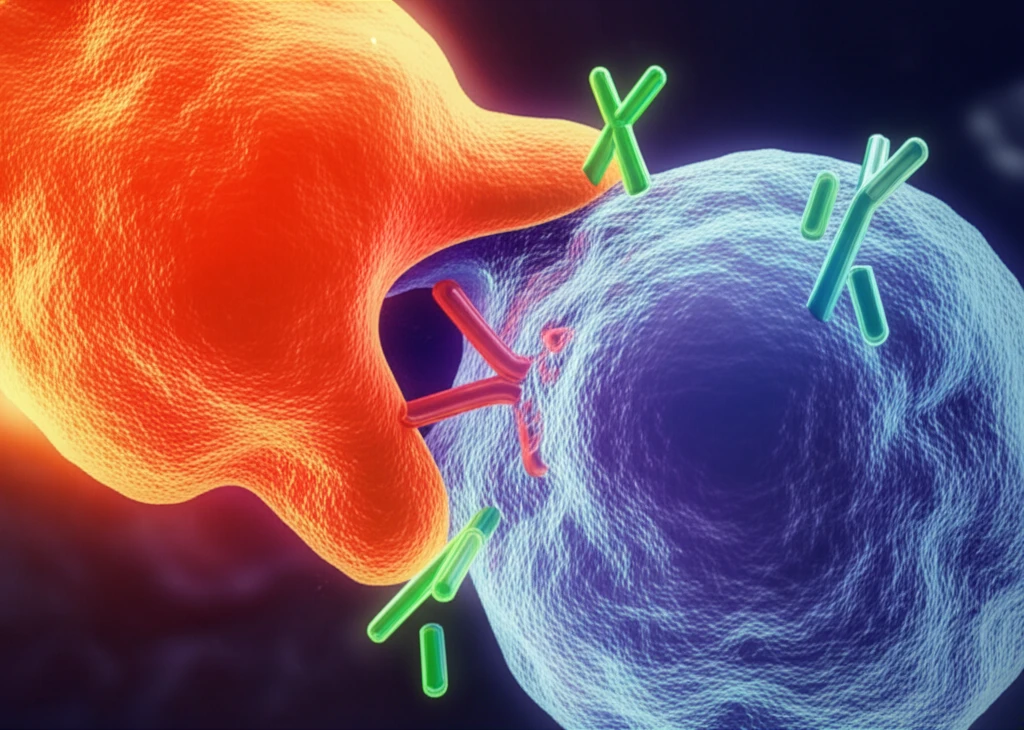
One particularly promising avenue is the CD47-SIRPa innate immune checkpoint. This checkpoint is formed by the interaction between CD47, a protein found on the surface of tumor cells, and SIRPa, an inhibitory receptor expressed on myeloid cells such as macrophages and granulocytes. CD47 acts as a "don't eat me" signal, preventing these immune cells from attacking and destroying cancer cells.
A recent study by Advani et al. marks a significant milestone in this field, providing the first-in-human data on the effects of an agent targeting the CD47-SIRPa interaction. This article delves into the findings of this study and explores the potential of myeloid immune-checkpoint inhibition as a novel approach to cancer therapy.
Unlocking the Potential of CD47-SIRPa Inhibition

The study by Advani et al. investigated the safety and efficacy of an anti-CD47 antibody, Hu5F9-G4, in combination with rituximab, an anti-CD20 antibody, in patients with non-Hodgkin lymphoma (NHL). The results were encouraging, demonstrating clinical responses with manageable toxicity levels. This combination therapy showed particular promise in patients with relapsed or refractory CD20-positive B cell NHL who had previously undergone multiple lines of therapy.
- A 36% complete response rate and a 14% partial response rate.
- Objective responses in both diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL) subtypes.
- Responses observed in patients with various DLBCL subtypes, including those with aggressive double-hit lymphomas.
- A 91% ongoing response rate among responders at the data cut-off point.
The Future of Cancer Therapy: A New Frontier
The success of the Hu5F9-G4 and rituximab combination raises important questions about the mechanism of action and potential for further improvement. While Hu5F9-G4 is known to inhibit the CD47-SIRPa interaction, its human IgG4 backbone could also contribute to its effects. Future research will need to explore the specific roles of different antibody isotypes and Fc regions in promoting anti-tumor activity.
Another key question is whether targeting SIRPa directly with anti-SIRPa antibodies could offer advantages over anti-CD47 antibodies. SIRPa has a more restricted expression pattern, potentially leading to fewer off-target effects. Furthermore, anti-CD47 antibodies may disrupt the recognition of other CD47 ligands, contributing to toxicity.
Despite these remaining questions, the study by Advani et al. represents a major step forward in the field of immuno-oncology. It demonstrates that targeting the CD47-SIRPa innate immune checkpoint is a safe and effective strategy for treating lymphoma, opening the door for further research and the development of novel cancer therapies.
