Liquid Crystal Breakthrough: Graphene Oxide Gets a Non-Polar Makeover
"Scientists have discovered a way to manipulate graphene oxide liquid crystals, opening doors to advanced materials and applications."
Graphene, a two-dimensional carbon material, has captivated scientists with its remarkable properties, paving the way for innovations in electronics, mechanics, and thermal applications. However, harnessing the full potential of graphene requires overcoming challenges in its production and dispersion. One promising avenue involves graphene oxide (GO), a derivative of graphene that can be chemically exfoliated in liquid phases, offering a pathway to large-scale production of graphene sheets.
GO possesses unique characteristics, including high colloidal stability in water due to the electrostatic repulsion of negatively charged oxygen groups. Notably, GO can form liquid crystal (LC) phases in water, presenting exciting opportunities for creating advanced 2D graphene-based composites. The challenge, however, lies in extending these LC phases to organic solvents, especially non-polar ones, which are crucial for various applications.
Recent scientific advancements have introduced a novel technique using poly(ionic liquid)s (PILs) to facilitate the dispersion of graphene oxide liquid crystals (GOLCs) from aqueous solutions into non-polar organic phases. This groundbreaking approach not only broadens the range of solvents in which GOLCs can exist but also opens new possibilities for material design and application.
The Science Behind the Breakthrough

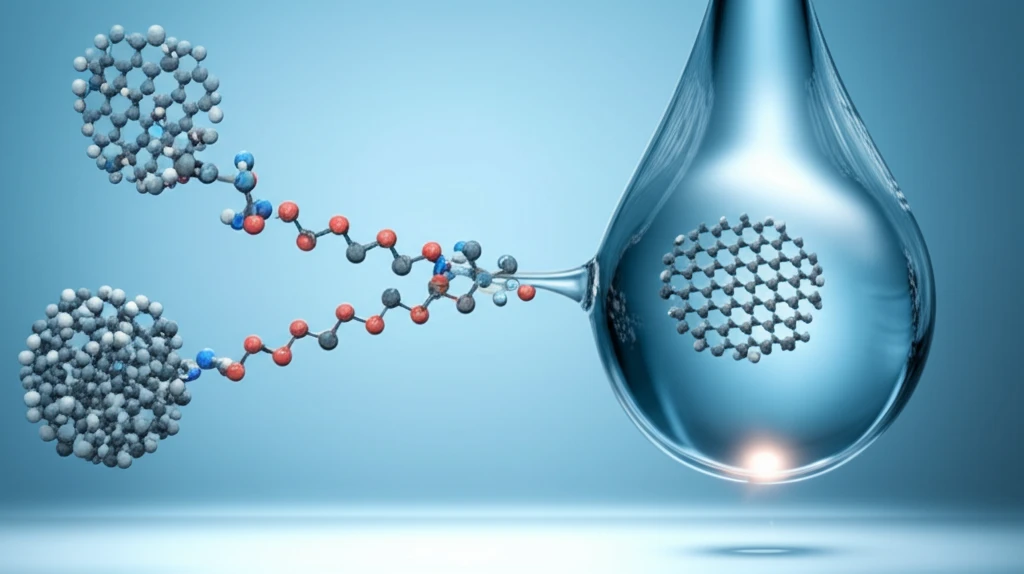
The key innovation involves using poly(ionic liquid)s (PILs), specifically a novel PIL called [PEP-MIM]DBS, to act as a phase-transfer agent. This agent enables the transportation of GO from water to organic solvents, all while preserving the GOLC phase. The PIL works by noncovalently interacting with GO, effectively decorating the GO nanosheets and preventing them from aggregating. This is crucial because once water is removed from GO, the sheets tend to clump together due to electrostatic and π-π interactions, making it difficult to redisperse them in organic solvents.
- Overcoming Aggregation: PILs prevent GO sheets from clumping together in non-polar solvents.
- Expanding Solvent Options: Previously limited to polar solvents, GOLCs can now exist in non-polar environments.
- Non-Covalent Interaction: The PILs interact with GO without forming strong chemical bonds, preserving the GO's structure.
- Versatile Applications: This method opens doors to new material designs and applications for graphene-based composites.
The Future of Graphene Oxide
This research marks a significant step forward in graphene oxide research, providing a practical method to prepare non-polar solvent-soluble GO sheets that can form LCs. By using PILs to decorate the GO sheets, scientists can now explore a wider range of applications for these materials, potentially leading to breakthroughs in areas such as advanced electronics, sensors, and composite materials. The ability to manipulate GO in non-polar solvents opens up new avenues for creating innovative materials with tailored properties and functionalities.
