Unlocking the Body's Defenses: How Cell 'Gatekeepers' Fight Off Deadly Infections
"Scientists Discover a Revolutionary 'Dock-and-Lock' Mechanism That Could Lead to New Treatments for Bacterial Infections"
Our bodies are constantly under siege from microscopic invaders. Among the most dangerous are bacteria, which can cause a wide range of illnesses, from minor infections to life-threatening conditions. But, our cells are not defenseless. They have sophisticated mechanisms in place to recognize and neutralize these threats.
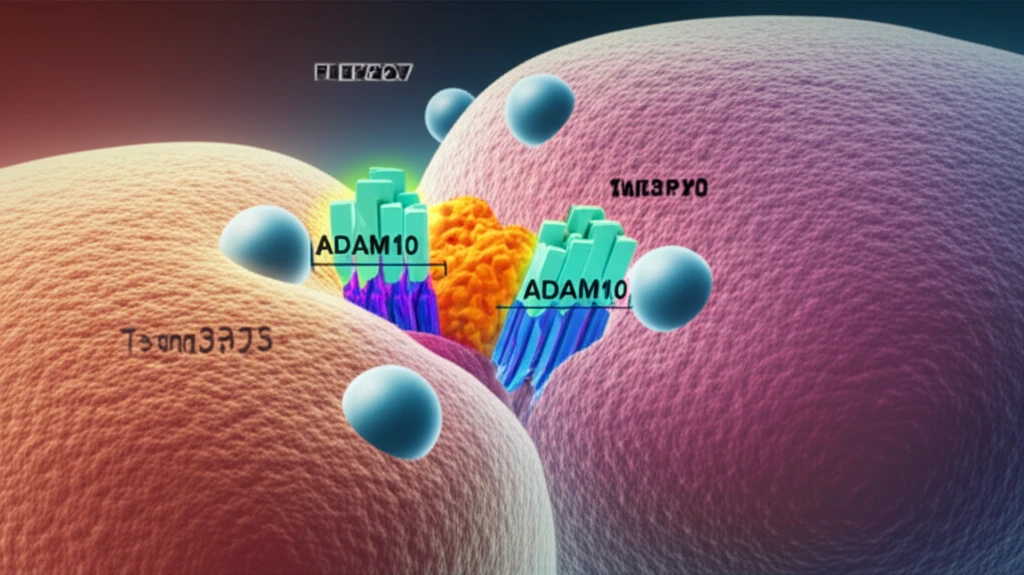
Recent research has unveiled a fascinating new strategy employed by our cells to combat one particularly nasty bacterium, Staphylococcus aureus, which causes staph infections. The study identifies a unique 'dock-and-lock' mechanism that allows cells to cluster a critical receptor, ADAM10, at junctions where cells meet. This clustering is key to fighting off the bacteria's toxins.
This breakthrough not only deepens our understanding of cellular defense but also opens doors to novel therapeutic approaches. By understanding how cells manage and deploy their defenses, scientists may be able to develop new treatments that can enhance the body's ability to fight off bacterial infections.
The 'Dock-and-Lock' Mechanism: A Cellular Security System

The research, published in Cell Reports, centers around a protein called ADAM10. ADAM10 acts as a cellular 'gatekeeper' for a-toxin from Staphylococcus aureus. The bacteria's a-toxin works by creating pores in our cell membranes, which can lead to cell damage and death. ADAM10 is the receptor on the surface of our cells that a-toxin uses to latch on.
- The Dock: The docking process is initiated by a protein called Tspan33. It binds to ADAM10, bringing it to the cell junctions.
- The Lock: PLEKHA7, acting as the lock, then secures ADAM10 at these junctions, forming a cluster.
The Future of Fighting Infections
This new understanding opens up exciting possibilities for future medical treatments. By targeting the 'dock-and-lock' mechanism, scientists could potentially develop therapies that either enhance or disrupt ADAM10 clustering. Enhancing clustering could boost the body's ability to fight off infections, while disrupting it could reduce the severity of infections. The research gives us a glimpse into the intricate world of cellular defense and provides a promising path forward in the ongoing battle against bacterial infections.
