Unlock Cellular Secrets: How SIRT7 Impacts Aging and Disease
"Discover the crucial role of SIRT7 in regulating cell function, with potential implications for cancer therapy and age-related conditions."
In the intricate world of cellular biology, proteins act as master regulators, orchestrating a myriad of processes that determine our health and longevity. Among these, Sirtuins—particularly SIRT7—have emerged as key players in maintaining cellular equilibrium. SIRT7, initially identified for its role in modifying histones within chromatin, is now recognized to influence a wider range of cellular activities. Recent research illuminates its function in ribosomal biogenesis, stress response, and, notably, its interaction with the CRL4 E3 ligase complex.
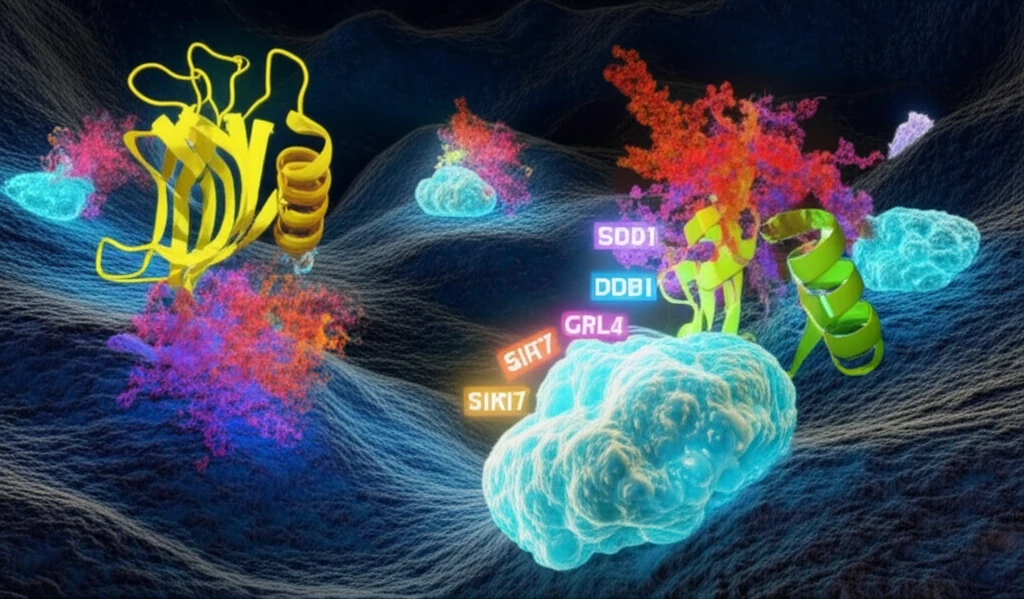
The CRL4 complex, essential for tagging proteins with ubiquitin to mark them for degradation or modification, is tightly controlled to ensure proper cellular function. This regulation is where SIRT7 steps in, acting as a deacetylase that directly impacts DDB1, a critical component of the CRL4 complex. The deacetylation of DDB1 by SIRT7 influences the activity of the CRL4 complex, affecting downstream cellular processes such as apoptosis and the cell's response to stress.
Understanding SIRT7's role in this complex interplay is not just an academic exercise; it has profound implications for how we approach cancer therapy and manage age-related diseases. By uncovering the mechanisms through which SIRT7 modulates the CRL4 complex, scientists are paving the way for targeted interventions that could restore cellular balance and improve health outcomes.
SIRT7's Role in Cellular Processes: A Deeper Dive

SIRT7, belonging to the Sirtuin family known for their NAD⁺-dependent deacetylase activity, has intrigued researchers due to its unique functions compared to other family members. Unlike its counterparts, SIRT7 predominantly resides in the nucleolus, the cell's ribosome factory, although it can also be found in the nucleoplasm. Its primary function involves ribosomal biogenesis, a process essential for protein synthesis and cellular growth. However, SIRT7's influence extends far beyond this singular role.
- Regulation of CRL4 Activity: SIRT7 directly deacetylates DDB1, a key adaptor protein in the CRL4 E3 ubiquitin ligase complex, impacting the complex's activity.
- Apoptosis Modulation: By influencing the CRL4 complex, SIRT7 affects the stability and activity of proteins like LATS1 and p73, which are crucial in initiating apoptosis.
- Stress Response: SIRT7 relocates within the cell under stress conditions, enhancing its control over DDB1 deacetylation and thus fine-tuning the cellular response.
The Future of SIRT7 Research: Implications for Therapy
As research into SIRT7 progresses, its therapeutic potential becomes increasingly evident. The ability of SIRT7 to influence cell apoptosis and stress response makes it a compelling target for cancer therapy. Drugs that modulate SIRT7 activity could enhance the effectiveness of existing treatments like Actinomycin D (ActD) and 5-Fluorouracil (5-FU), potentially improving outcomes for patients with various cancers. Furthermore, understanding how SIRT7 contributes to age-related diseases could pave the way for interventions that promote healthier aging.
