DNA Primase's Redox Role: New Insights into Enzyme Activity
"A fresh look at the structure of the iron-sulfur cluster domain in DNA primase challenges previous assumptions about its redox function and suggests alternative explanations for enzyme activity."
The process of DNA replication relies on a complex interplay of enzymes, each with a specific role. Among these, DNA primase is responsible for synthesizing short RNA primers that initiate DNA synthesis. Recent research by O'Brien et al. (1) proposed that the iron-sulfur cluster within primase has a redox function, influencing its activity.
The study suggested that the redox state of this cluster acts as a switch, modulating DNA binding and primer termination. The proposed mechanism involved electron transfer through the DNA helix, facilitated by specific tyrosine residues in primase. However, this model hinges on a particular structure of the primase domain, which has been called into question.
This article delves into a structural re-evaluation of the primase domain, revealing a potentially misfolded structure in the original study. We will explore how the corrected structure alters the understanding of the tyrosine-mediated electron transfer pathway and offers alternative explanations for primase's function in DNA replication.
The Misfolded Structure and Its Implications

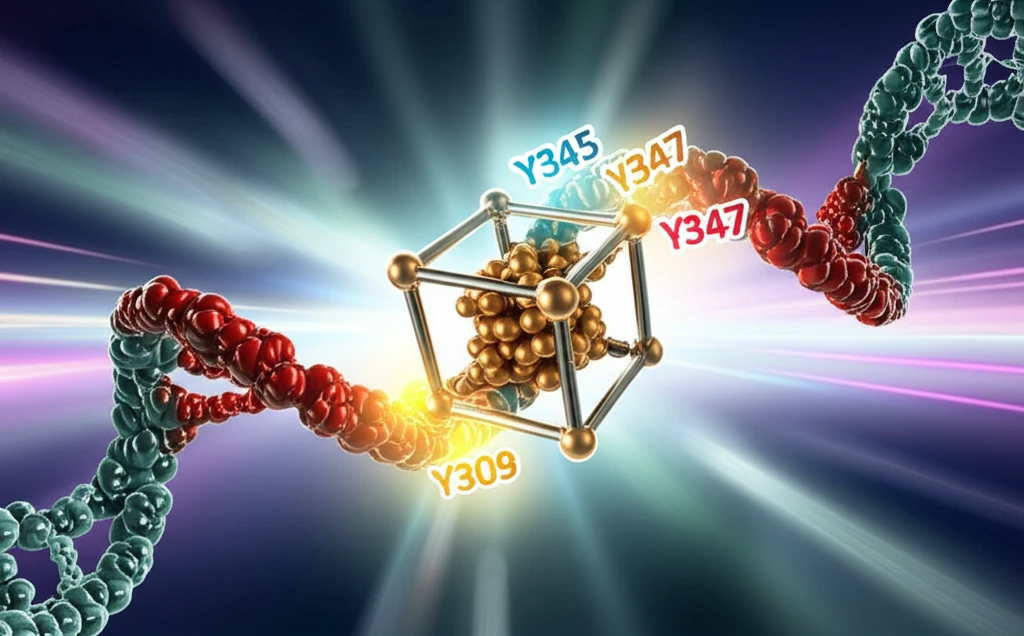
O'Brien et al.'s (1) hypothesis of the Fe-S cluster's redox activity hinges on a specific structure of the primase domain (p58C). This structure, however, contains a local misfolding—amino acids 318 to 360 adopt a beta-hairpin conformation instead of the alpha-helical structure observed in other structures of yeast and human p58C (4, 5). This discrepancy is likely due to a single-point mutation (I271S) present in the p58C construct used in the original study (3).
- Incorrect Folding: The beta-hairpin conformation alters the spatial arrangement of critical amino acids.
- Increased Distance: The distance between tyrosine residues increases, potentially hindering electron transfer.
- Non-conserved Residue: Tyrosine 309, a key component of the proposed electron wire, is not conserved across all eukaryotic primases.
Reassessing the Redox Role of Primase
Given the structural concerns and the alternative explanation for the mutagenesis data presented by O'Brien et al., it's important to reconsider the proposed redox role of the Fe-S cluster in primase. The evidence supporting this role appears less definitive when viewed through the lens of the corrected protein structure.
While the idea of a redox switch in primase is intriguing, further experimentation is needed to confirm this mechanism. Future studies should focus on validating the electron transfer pathway and directly measuring the redox potential of the Fe-S cluster in the context of the correctly folded primase structure.
Understanding the precise function of the iron-sulfur cluster in primase remains an open question. By considering the accurate protein structure and exploring alternative explanations, researchers can continue to unravel the complexities of DNA replication and the roles of key enzymes like primase.
