Decoding Immunity: How Cell Location and Triggers Shape T Cell and Prostate Cell Behavior
"Unlocking the secrets of intracellular processes for targeted therapies in autoimmune diseases and cancer."
Our immune system's T cells and the cells within our organs, like the prostate, don't operate in isolation. Their behavior is heavily influenced by their surroundings and the signals they receive. Recent research is shedding light on how these local interactions shape cell function, with significant implications for understanding and treating diseases like autoimmune disorders and cancer.
Two key areas of focus are how T cells are activated to fight off threats and how prostate cells maintain healthy function. In T cells, the process of 'complement C3-licensing' is crucial for launching effective immune responses. In prostate cells, intracellular C3a plays a vital role in maintaining normal cell behavior.
This article delves into the latest findings on these processes, exploring how cell location and specific triggers dictate cell behavior. By understanding these intricate mechanisms, we can potentially develop more targeted and effective therapies for a range of diseases.
The Role of Location: T Cell Activation and the Importance of LFA-1

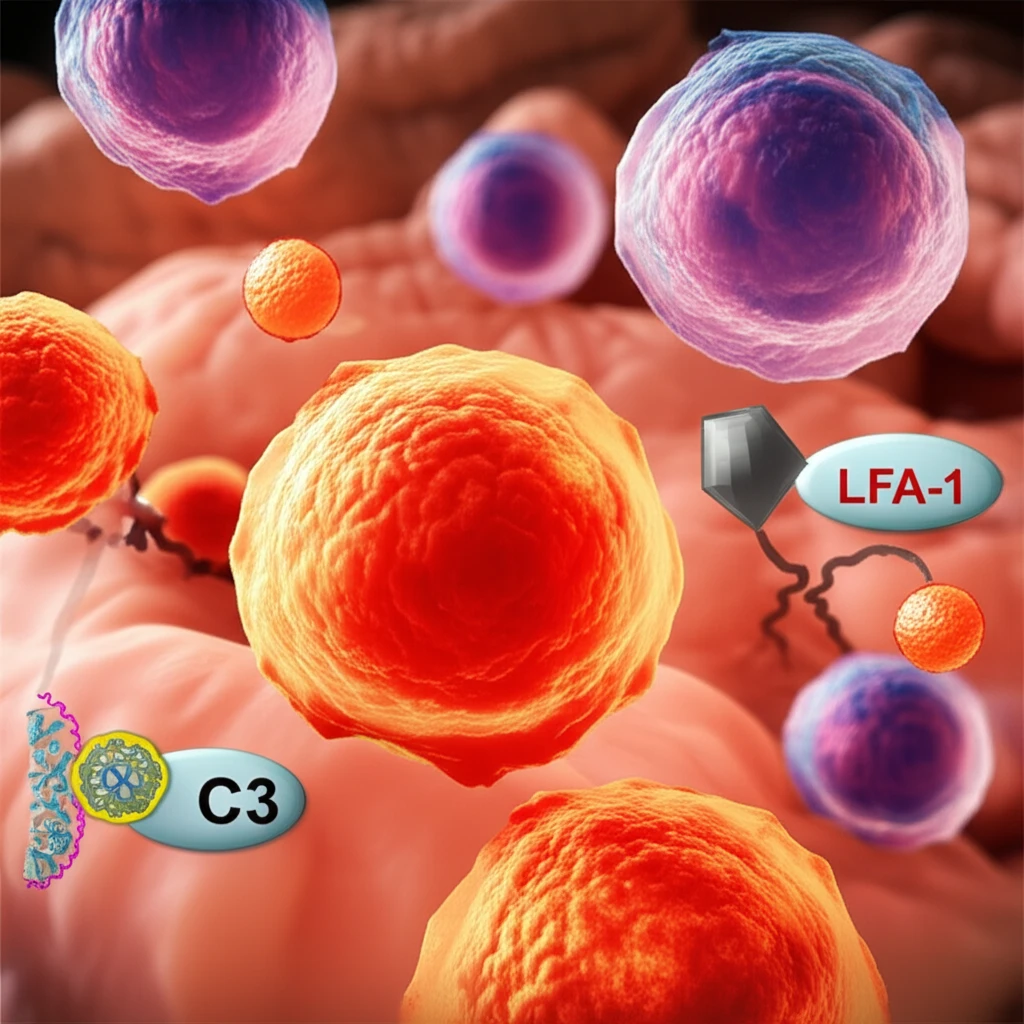
T cells are essential for orchestrating immune responses. Their activation and ability to launch an effective attack depend on a complex interplay of signals. One crucial aspect of this activation is the 'complement C3-licensing' process, which is necessary for successful Th1 responses – a type of immune response vital for fighting intracellular pathogens and tumors.
- LFA-1's Key Role: The integrin LFA-1, which helps T cells move out of blood vessels (extravasation), plays a crucial role. When LFA-1 on T cells is stimulated by ICAM-1, it triggers strong C3 gene expression.
- AP-1 Dependence: This C3 gene expression relies on the AP-1 pathway, a key regulator of gene expression in cells.
- Enhanced IFN-γ Production: In conjunction with T-cell receptor stimulation, LFA-1 activation increases the T cell's capacity to produce IFN-γ, a critical cytokine for Th1 responses.
- Blocking LFA-1 Impairs Activation: Blocking the LFA-1/ICAM-1 interaction during T cell migration prevents C3 expression and Th1 induction.
- LFA-1 Deficiency Leads to Impaired Responses: T cells from LFA-1 deficient patients fail to increase C3 transcription and generate Th1 responses.
- Restoring C3 Restores Function: Introducing intracellular C3 back into these cells restores their ability to induce Th1 responses.
- 'Transmigration-Experienced' Cells: T cells in inflamed tissues show increased C3 expression and augmented IFN-γ production.
- In Vivo Confirmation: Imaging studies confirm that C3 gene transcription is induced in T cells entering sites of infection.
Intracellular C3a and Prostate Cell Homeostasis: A Delicate Balance
While circulating complement proteins protect against infections, intracellular complement C3 also plays a vital role within cells. In prostate cells, intracellular C3a regulates proliferation through the mTOR/glycolysis pathway. Disruptions in this system can contribute to abnormal cell function, potentially leading to cancer.
Studies have revealed that while normal prostate cells express 'homeostatic' C3a, cancer cells often exhibit reduced or absent C3/C3a expression. The extent of this reduction correlates with worsening Gleason scores, a measure of cancer aggressiveness. In slow-growing prostate cancer cells, C3a is present, but it's absent in more aggressive, androgen-insensitive cells.
Furthermore, inhibiting C3 in benign prostate cells increases cell proliferation, while restoring intracellular C3a curbs cell growth in certain cancer cells. Animal studies have shown that C3a deficiency leads to malignant transformation in the prostate. These findings suggest that intracellular C3 negatively controls prostate epithelial cell proliferation, highlighting a contrast to T cells, where C3a drives proliferation. Though C3 activation is ubiquitous, its activity is cell type-specific, pointing to context-dependent effects and opening therapeutic avenues.
