Unlocking Lower Back Pain: How the Tiny Vacuoles in Your Spine Hold the Key to Disc Health
"Could Understanding These Microscopic Structures Revolutionize Treatment for Degenerative Disc Disease?"
Lower back pain (LBP) stands as a global health burden, impacting a significant portion of the population and leading to disability. Intervertebral disc degeneration (IVDD), a condition affecting the discs that cushion the vertebrae, is a major contributor to LBP. Unfortunately, treatments often focus on symptom relief instead of targeting the root causes of IVDD.
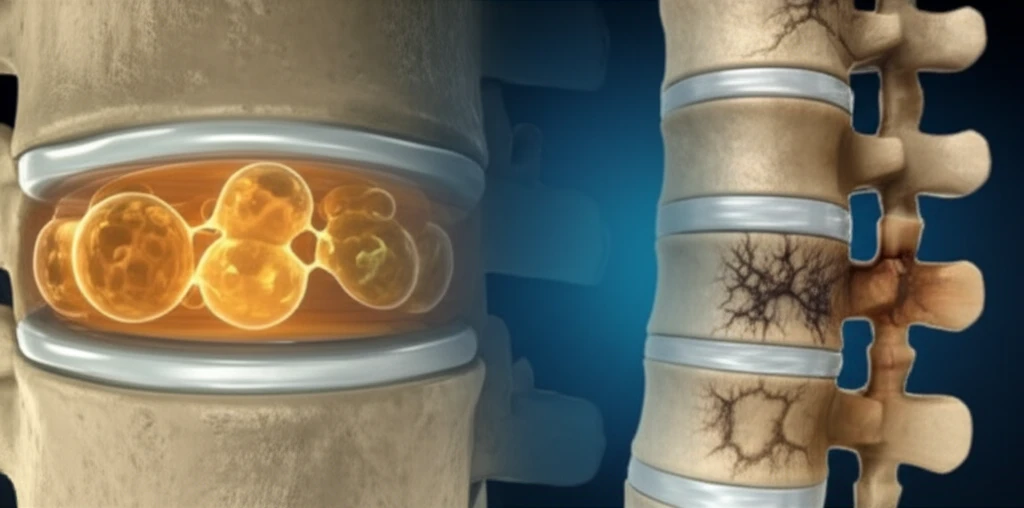
The intervertebral disc (IVD) comprises of nucleus pulposus (NP), annulus fibrosus (AF), and cartilaginous endplate (CEP). The nucleus pulposus (NP), the disc's gelatinous core, originates from the embryonic axial notochord. Fascinatingly, cells within the NP, known as notochordal cells, are characterized by unique, large cytoplasmic vacuoles. These vacuoles are not just empty spaces, they are organelles of special functions.
This article delves into the formation, function, and eventual decline of these notochordal vacuoles, exploring their crucial role in maintaining disc health and their potential as a target for regenerative therapies.
The Mighty Vacuole: What Do These Structures Do?

Notochordal cells (NNPCs) are unique because of their prominent cytoplasmic vacuoles, which can occupy a large portion of the cell's volume. These aren't just random bubbles; they're membrane-bound structures with specialized functions. NNPCs have capabilities, including:
- Generating new chondrocyte cells
- Attracting chondrocytes to the NP
- Secreting growth factors that rejuvenate cells
- Stimulating chondrogenesis
- Suppressing infiltration of nerve or blood vessels
- Suppressing chondrocyte death in the harsh disc niche
The Future of Disc Regeneration: Targeting the Vacuole
Notochord vacuolation is vital to embryonic development and is orchestrated by vacuolating signals. Notochord cells are a source of mechanical support that are required for the elongation of growing embryos, and for morphogenesis of the vertebral column. Once the cells are squeezed into the center of the IVD, mechanical stress and an avascular nature of the site can increase exhaustion of vacuoles and promote CNPC transformation. Premature de-vacuolation and degeneration can be better understood, by understanding notochord vacoules.
Given the active and versatile role of notochord vacuoles in both embryonic notochord cells and postnatal NNPCs, preservation of vaculoating signals could slow down IVD degeneration.
However, to understand how mammalian vertebral collumns are formed more research is required. This will help validate signaling networks and biological functions of notochord vacuolation.
