IGF-1's Secret Weapon: How PKM2 Phosphorylation Drives Cancer Cell Growth
"Unlocking the AKT-PKM2 Connection for Novel Cancer Therapies."
Insulin-like Growth Factor 1 (IGF-1) is a key player in the microenvironment surrounding tumors. It acts like a signal booster, encouraging cancer cells to grow and survive. While IGF-1's role in cancer has been known, the precise mechanisms are still being unraveled.
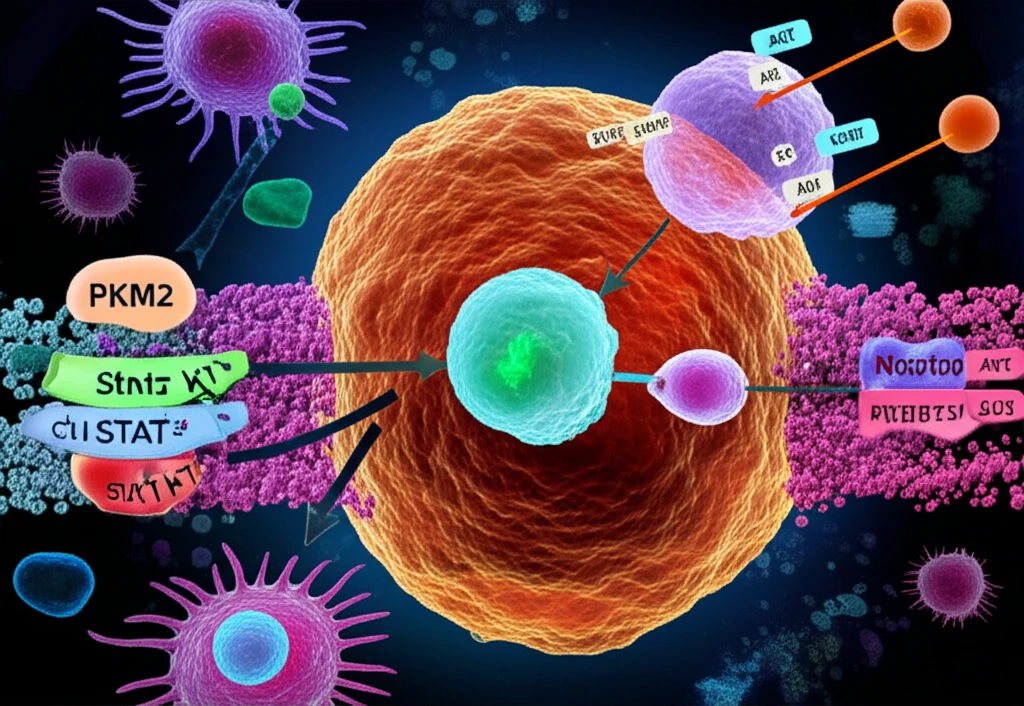
New research sheds light on a critical piece of this puzzle: a protein called pyruvate kinase muscle type 2 (PKM2). PKM2 undergoes changes after it's created (post-translational modifications) in response to signals from its surroundings, and these changes can significantly impact cancer cell behavior. Scientists have discovered how PKM2 gets activated in response to IGF-1, making it a potential weak spot for future cancer therapies.
This article dives into how AKT, another important protein in cell signaling, directly interacts with PKM2. This interaction leads to PKM2 phosphorylation, a process that's essential for PKM2 to move into the nucleus of cancer cells and drive tumor growth. Understanding this process opens doors to novel strategies for combating cancers driven by IGF-1 signaling.
The AKT-PKM2 Connection: A New Target for Cancer?

The study highlights a specific chain of events: AKT, a protein kinase, directly interacts with PKM2 and phosphorylates it at a specific location (Ser-202). This phosphorylation is a crucial step that allows PKM2 to move into the cell's nucleus when stimulated by IGF-1. Once inside the nucleus, PKM2 gets cozy with another protein called STAT5A. This partnership boosts the production of cyclin D1, a protein that encourages cell division and growth. It’s like PKM2 is acting as a key to unlock STAT5A's potential to accelerate cancer cell growth under IGF-1's influence.
- Blocking PKM2: When PKM2 was suppressed, the cancer cells' ability to grow in response to IGF-1 was significantly reduced.
- AKT Inhibition: Preventing AKT from activating blocked PKM2's movement to the nucleus.
- STAT5A's Role: If cells lacked PKM2, simply adding more STAT5A couldn't restore the cancer cells' growth response to IGF-1. However, if PKM2 was reintroduced, the cells regained their ability to grow when exposed to IGF-1.
Implications and Future Directions
This research highlights the significance of PKM2 as a potential therapeutic target. By understanding how AKT activates PKM2, scientists can develop drugs that disrupt this interaction, potentially slowing down or stopping cancer cell growth.
While this study focused on lung cancer cells, the IGF-1/AKT/PKM2 pathway is relevant in various cancer types. This makes the findings broadly applicable and opens avenues for developing treatments that target multiple cancers.
Further research is needed to fully understand how PKM2 interacts with STAT5A and regulates gene expression. Unlocking these details could lead to even more precise and effective cancer therapies. The discovery that PKM2 phosphorylation at Ser-202 is crucial for its interaction with STAT5 offers a specific target for drug development.
