Cracking the Code: How Scientists Are Unlocking the Secrets of Cellular Transport
"Groundbreaking research sheds light on ion selectivity in Vibrio cholerae, paving the way for new antimicrobial strategies and a deeper understanding of cellular function."
Imagine your cells as bustling cities, constantly moving essential goods and waste products across their membranes. Cation-proton antiporters are the gatekeepers of these cellular cities, meticulously controlling the flow of ions like sodium, potassium, and lithium to maintain the delicate balance required for life. In the marine bacterium Vibrio cholerae, survival hinges on these antiporters, particularly a trio known as NhaP. But one of these, NhaP2, has a unique quirk: it can bind lithium but doesn't readily exchange it for protons, a puzzle that has captivated researchers.
New research is diving deep into the intricate workings of Vc-NhaP2, aiming to understand how it distinguishes between different ions. Scientists are employing sophisticated computer modeling techniques and experimental methods to map the precise structure of this protein and pinpoint the regions responsible for its selective behavior. The goal? To unlock the secrets of ion selectivity and potentially develop new ways to combat V. cholerae infections.
Vc-NhaP2's ability to withstand acidic conditions is crucial for its survival. Researchers believe that understanding and targeting Vc-NhaP2 could pave the way for novel antimicrobial strategies specifically designed to disrupt V. cholerae's ability to thrive in the human body.
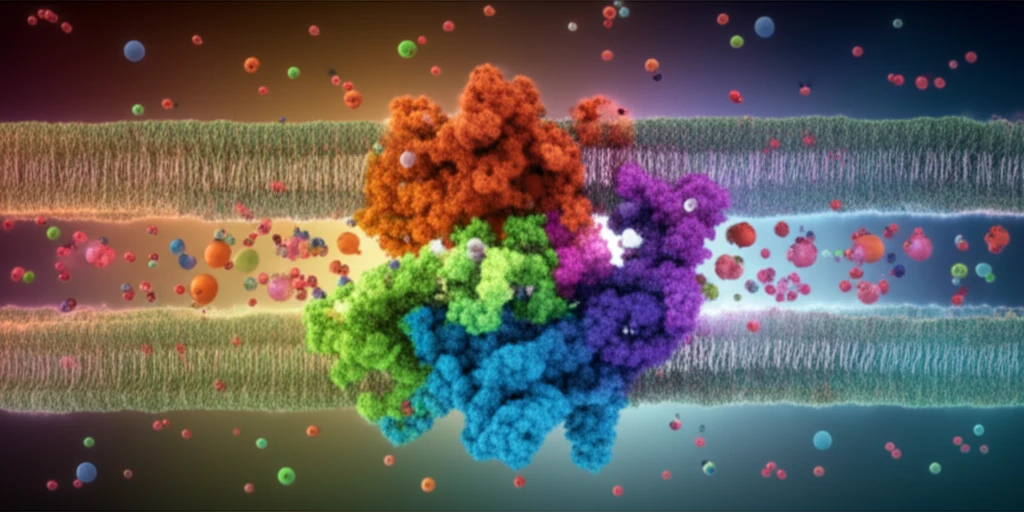
Decoding the Structure: A 3D Model of Vc-NhaP2

To visualize the inner workings of Vc-NhaP2, researchers turned to computational modeling. Using software like Phyre2 and Rosetta, they created a detailed 3D model of the protein based on its amino acid sequence and comparison to similar proteins with known structures. This model revealed a complex architecture, with key components:
- Cation-Binding Pocket: A central cavity formed by residues from different transmembrane segments, poised to capture and manipulate ions.
- Transmembrane Pathway: A channel-like structure composed of amino acids from TMS IX, X, and XII, acting as a selective gateway for ions.
The Future of Cellular Transport Research
This research marks a significant step forward in our understanding of cellular transport mechanisms. By combining computational modeling with experimental validation, scientists are gaining unprecedented insights into the intricate workings of membrane proteins. The knowledge gleaned from this study could have far-reaching implications, from the development of targeted antimicrobials to a deeper appreciation of the fundamental processes that sustain life.
