Cracking Chirality: How a New ZIF Membrane Could Revolutionize Chemical Separations
"Scientists have developed a homochiral zeolitic imidazolate framework (ZIF) membrane that significantly improves chiral separation, opening doors for more efficient drug manufacturing and food chemistry."
Chirality, the property of molecules existing as non-superimposable mirror images, plays a pivotal role in various fields, from medicine and life sciences to food chemistry and drug manufacturing. Each enantiomer of a chiral molecule can exhibit vastly different biological activities, making the ability to separate them crucial. Traditional methods such as spontaneous crystallization and enzymatic kinetic resolution are often complex and energy-intensive.
In recent years, metal-organic frameworks (MOFs) have emerged as promising materials for molecular separation due to their tunable pore sizes, high surface areas, and good adsorption properties. However, creating MOF membranes with the ability to efficiently separate chiral molecules has remained a significant challenge, requiring precise control over the introduction of chiral functionalities.
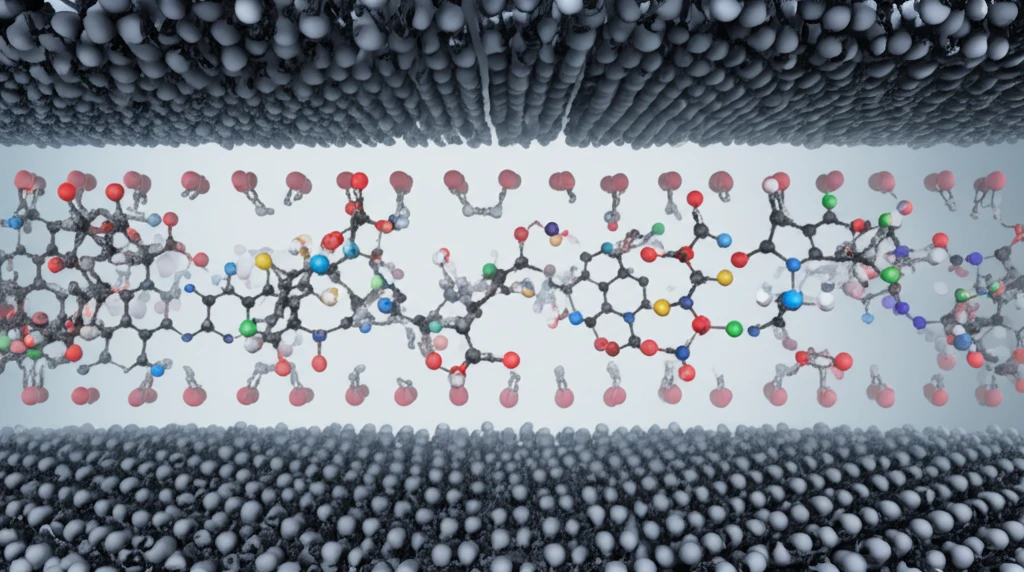
Now, researchers have announced a breakthrough in the creation of a homochiral zeolitic imidazolate framework-8 (ZIF-8) membrane, modified with the natural amino acid L-histidine (L-His). This innovative membrane demonstrates exceptional selectivity in separating the enantiomers of 1-phenylethanol, achieving a high enantiomeric excess value of up to 76%.
The Innovation: L-His-ZIF-8 Membrane

The research team successfully synthesized a homochiral L-His-ZIF-8 membrane using a contra diffusion method, where L-histidine was incorporated into the ZIF-8 framework. This membrane exhibited a remarkable preference for the R-enantiomer of 1-phenylethanol over the S-enantiomer. Selectivity arises from specific interactions between the S-enantiomer and the chiral MOF framework, paving the way for highly efficient chiral separation.
- Energy-dispersive X-ray spectroscopy (EDX) showed the presence of oxygen atoms associated with the carboxy group of L-His.
- Fourier-transform infrared (FTIR) spectroscopy detected adsorption peaks corresponding to the carboxy and amine groups of L-His.
- X-ray photoelectron spectroscopy (XPS) identified the binding energy of the carboxylic group in L-His-ZIF-8.
- Solid-state nuclear magnetic resonance (NMR) revealed additional peaks attributed to L-His.
Future Implications and Sustainability
This breakthrough in creating a highly selective homochiral MOF membrane opens up new avenues for chiral separation. The L-His-ZIF-8 membrane demonstrates exceptional selectivity and stability, making it a promising candidate for practical applications in the pharmaceutical, chemical, and food industries. It represents a significant step toward more efficient, sustainable, and cost-effective chiral separation processes, potentially revolutionizing the way we produce essential compounds and drugs. The successful incorporation of a natural amino acid into the MOF framework highlights the potential for designing even more sophisticated and biocompatible membranes for a wide range of separation challenges.
