Cellular 'Dock-and-Lock': How Junctions Can Trigger Deadly Toxin Reactions
"Scientists uncover a surprising mechanism where cell-cell junctions amplify the danger of a common bacterial toxin, paving the way for targeted therapies."
Our bodies are constantly under siege. From the moment we’re born, we’re battling microscopic invaders – bacteria, viruses, and other pathogens that seek to colonize and wreak havoc. One of our primary defenses is our epithelial cells. These cells line our surfaces, from the skin to the delicate linings of our lungs and digestive tract, acting as a crucial barrier against the outside world.
But even the best defenses can be breached. Many bacteria have evolved sophisticated weapons to overcome our cellular fortifications. Among the most potent of these weapons are pore-forming toxins. These toxins, secreted by bacteria, target the membranes of our cells, creating holes that disrupt their function and ultimately lead to cell death. A particularly notorious example is alpha-toxin, produced by the common bacterium Staphylococcus aureus (S. aureus), responsible for a range of infections, from skin irritations to life-threatening sepsis.
For years, scientists have been working to understand exactly how alpha-toxin inflicts its damage. They've identified key host cell receptors – molecules on the surface of our cells that the toxin binds to. But a recent discovery has revealed a surprising twist: our own cell-cell junctions, the very structures that hold our tissues together, can actually amplify the effects of this deadly toxin. The new understanding could be critical for designing more effective therapies to combat S. aureus infections.
How Cell Junctions Escalate the Toxin's Impact

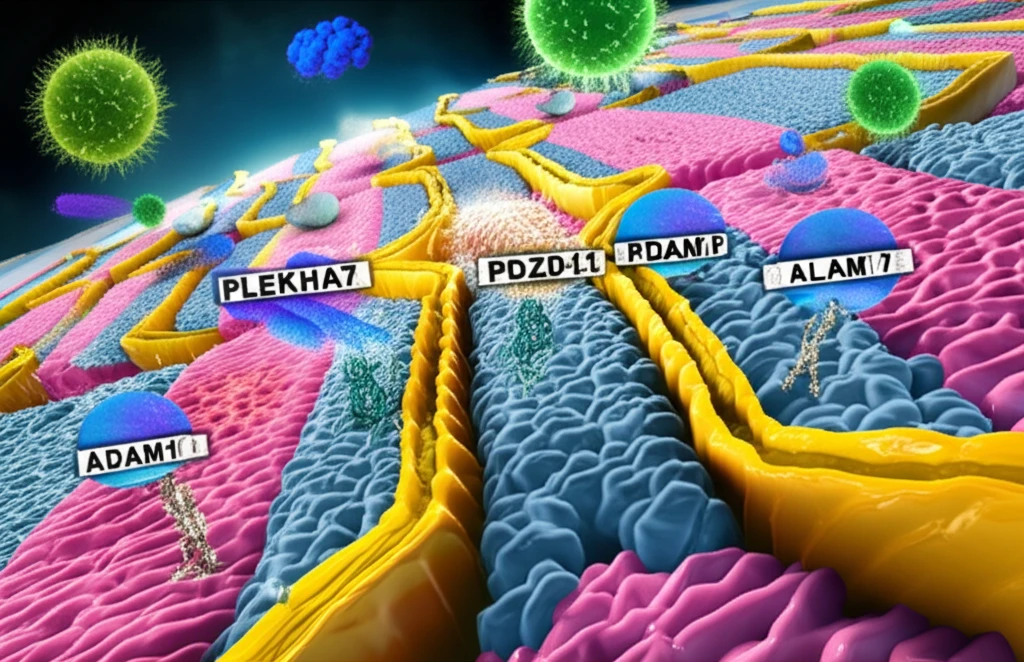
Cell junctions aren't just static structures; they're dynamic hubs of activity, packed with a variety of proteins that regulate cell adhesion, signaling, and even immune responses. Among these proteins, PLEKHA7, PDZD11, and afadin have emerged as key players in how cells respond to toxins. While scientists knew that these proteins were somehow involved in how our cells die from S. aureus, the exact process of junctions promoting toxicity had remained unclear until recently.
- Docking ADAM10: The transmembrane partner Tspan33 docks ADAM10 to junctions through its cytoplasmic C terminus binding to the WW domain of PLEKHA7 in the presence of PDZD11.
- Locking ADAM10: ADAM10 is locked at junctions through binding of its cytoplasmic C terminus to afadin.
- Efficient Pore Formation: Junctionally clustered ADAM10 supports the efficient formation of stable toxin pores.
- Removal of Toxins: Loss of PLEKHA7-PDZD11 leads to toxin pore removal by endocytosis and cell survival.
A New Path to Fighting Infections
This discovery of the 'dock-and-lock' mechanism has significant implications for how we approach treating S. aureus infections. Rather than simply targeting the toxin itself, a potential approach involves disrupting the interaction between PLEKHA7 and ADAM10 at cell junctions. By preventing the clustering of ADAM10, cells could be rendered less susceptible to alpha-toxin, giving the body's natural defenses a better chance to clear the infection. Future research will focus on developing drugs that specifically target the PLEKHA7-PDZD11-ADAM10 complex, offering a more targeted and potentially less toxic approach to combating S. aureus and other bacterial pathogens.
