AML Breakthrough: Can a New Antibody Construct Improve Treatment Outcomes?
"Research suggests a bispecific antibody targeting CD33 could enhance the fight against acute myeloid leukemia by attacking both cancer cells and immune-suppressing cells."
Acute myeloid leukemia (AML) remains a formidable challenge in adult oncology. Despite advancements in treatment, the five-year survival rate lingers below 30%, highlighting the urgent need for innovative therapeutic strategies. Recent research has begun to illuminate the critical role of immune evasion in AML progression and relapse, suggesting that harnessing the immune system could be key to improving patient outcomes.
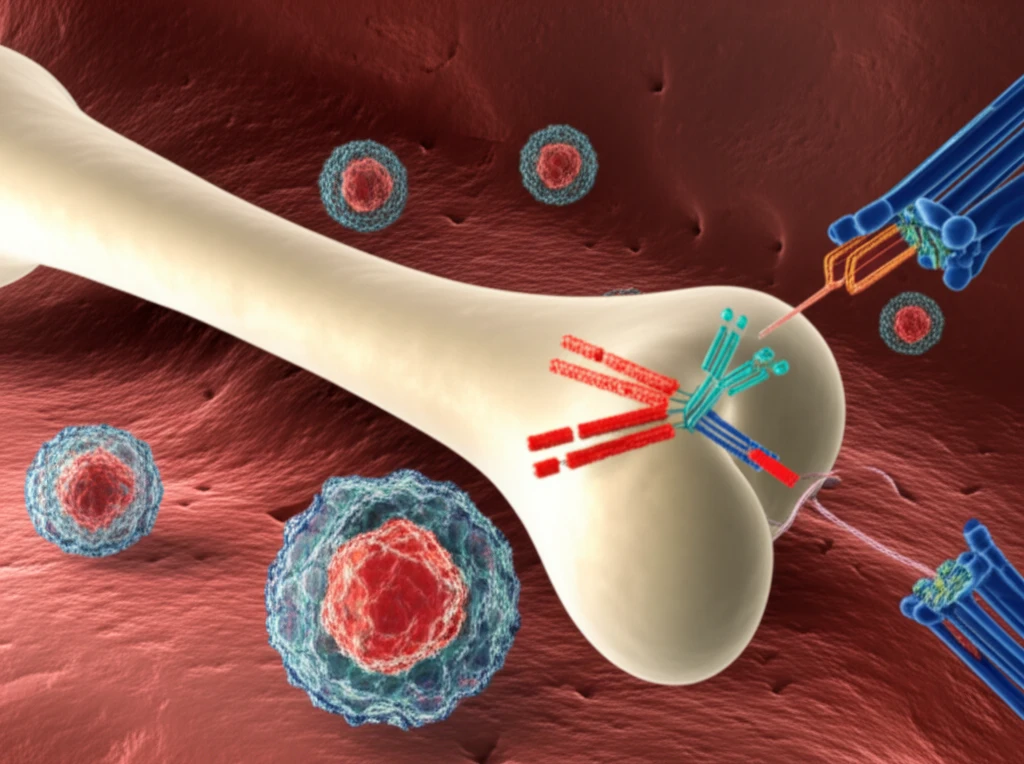
Among the factors contributing to immune evasion, myeloid-derived suppressor cells (MDSCs) have emerged as significant players. These cells, characterized by their ability to suppress T-cell function, create an environment that shields leukemia cells from immune attack. The presence of MDSCs in AML patients has been linked to poorer responses to therapy and increased risk of relapse, making them an attractive target for novel interventions.
Now, a new study offers a promising approach to targeting MDSCs in AML. Researchers have investigated a CD33/CD3-bispecific antibody construct, which redirects T-cells to eliminate both CD33-expressing AML cells and MDSCs. This dual-targeting strategy holds the potential to overcome immune suppression and enhance the effectiveness of AML therapy. Let's delve into the details of this research and its implications for the future of AML treatment.
What are Myeloid-Derived Suppressor Cells (MDSCs) and Why Do They Matter in AML?

MDSCs are a diverse population of immune cells that share the ability to suppress T-cell responses. In AML, these cells accumulate in the bone marrow and peripheral blood, creating an immunosuppressive environment that protects leukemia cells from immune attack. MDSCs achieve this suppression through various mechanisms, including the production of immunosuppressive molecules like indoleamine-2,3-dioxygenase (IDO) and arginase.
- Reduced response to chemotherapy
- Increased risk of relapse
- Impaired T-cell function
- Suppression of anti-tumor immunity
Future Implications for AML Treatment
The study's findings suggest that AMG 330 may offer a dual benefit in AML treatment by directly targeting leukemia cells and simultaneously eliminating immunosuppressive MDSCs. This approach could potentially overcome a significant barrier to effective immunotherapy in AML and improve patient outcomes. Additional research is needed to validate these findings in clinical trials and to explore the potential of combining AMG 330 with other immunotherapeutic strategies.
