Unlocking the Mystery: How Glucocorticoids, Angptl4, and Ceramides Impact Insulin Resistance
"A groundbreaking study reveals the intricate connection between glucocorticoid exposure, Angptl4, and ceramide production in driving insulin resistance, offering potential new therapeutic targets."
Insulin resistance is a growing health concern, acting as a major precursor to type 2 diabetes and cardiovascular diseases. While the role of glucocorticoids—hormones essential for regulating various bodily functions—in inducing insulin resistance has long been recognized, the precise mechanisms have remained elusive.
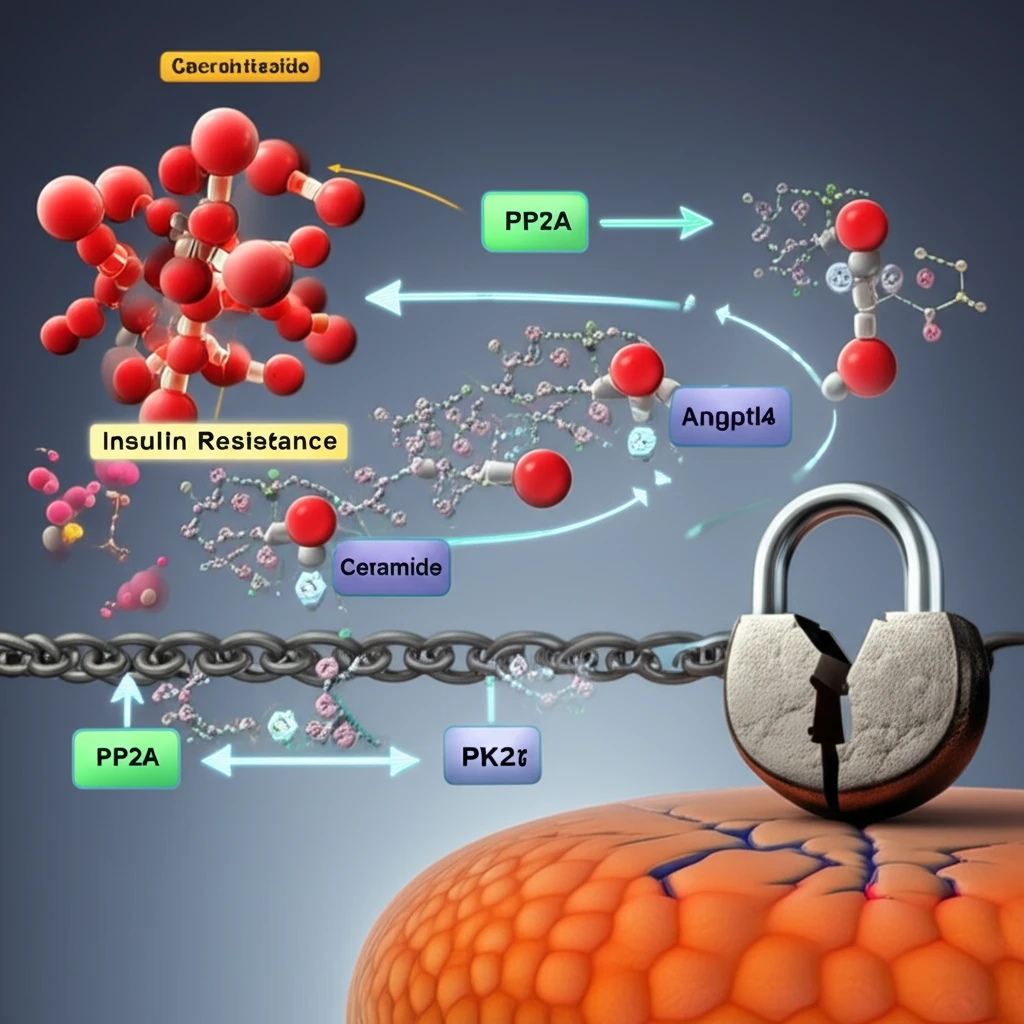
Emerging research has shed light on a critical pathway involving Angptl4 (angiopoietin-like 4), a protein regulated by glucocorticoids, and ceramides, a class of lipids. This axis appears to play a pivotal role in how glucocorticoids trigger insulin resistance, offering new targets for therapeutic intervention.
This article explores the groundbreaking study that unveils the intricate relationship between glucocorticoids, Angptl4, and ceramides, delving into the molecular mechanisms that drive insulin resistance. By understanding this complex interplay, we can pave the way for innovative strategies to combat metabolic disorders and improve overall health.
The Glucocorticoid-Angptl4-Ceramide Connection: A Deep Dive

The study, conducted by researchers at the University of California, Berkeley, focused on the effects of chronic glucocorticoid exposure on insulin resistance. They discovered that Angptl4, a glucocorticoid target gene, plays a crucial role in mediating glucocorticoid-induced lipolysis (the breakdown of fats) in white adipose tissue (WAT).
- Angptl4: A key protein that mediates glucocorticoid-induced lipolysis in white adipose tissue.
- Ceramides: A class of lipids whose hepatic concentrations increase with glucocorticoid treatment, impacting insulin sensitivity.
- PP2A and PKCζ: Downstream effectors of ceramide that play a role in insulin resistance.
Implications and Future Directions
This study unveils the critical role of Angptl4 in glucocorticoid-augmented hepatic ceramide production, which ultimately induces whole-body insulin resistance. These findings offer valuable insights into the pathogenesis of metabolic disorders and identify potential targets for therapeutic interventions. Further research is needed to fully elucidate the complex interplay between glucocorticoids, Angptl4, ceramides, and other signaling pathways involved in insulin resistance.
