Enzyme's Surprising Twist: How VioC Handles Mirror-Image Molecules
"Scientists discover VioC's unexpected ability to transform D-arginine, opening new doors in enzyme research and drug development."
Enzymes, the workhorses of biological systems, are known for their precise selectivity. Like a lock and key, they typically interact with specific molecules to catalyze reactions. However, recent research has illuminated a fascinating exception to this rule, challenging our understanding of enzyme behavior. This study uncovers how the enzyme VioC, traditionally known for processing L-arginine, can also transform D-arginine, a mirror image of its usual target.
This unexpected flexibility could lead to new ways of creating valuable compounds, designing medications, and understanding the subtle chemical transformations that underpin life itself. This article explores the innovative work of researchers who discovered VioC's versatility and the potential implications of their findings.
The traditional view of enzymes is being challenged. Scientists are finding that some enzymes can perform outside of their typical functions when presented with slightly different molecules. This 'promiscuity' can be a source of new chemical reactions and products, which has exciting implications for the fields of biotechnology and medicine.
VioC's Unexpected Transformation of D-Arginine: What Does It Mean?

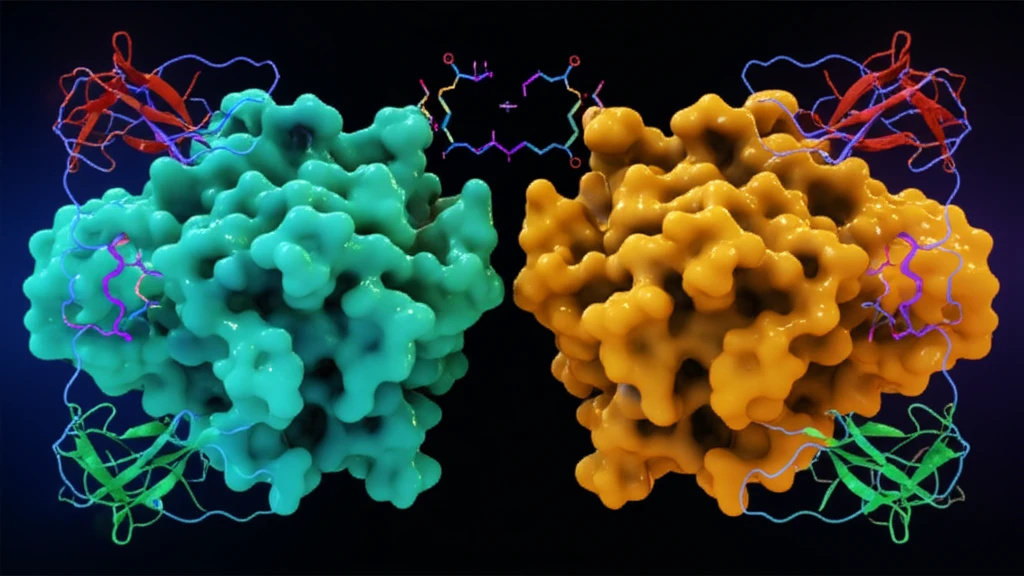
The study, led by researchers at the Pennsylvania State University, reveals that VioC, an enzyme involved in the biosynthesis of viomycin, a compound with antibiotic properties, can catalyze an unusual reaction with D-arginine. Normally, VioC acts on L-arginine, adding a hydroxyl group at a specific location. However, when presented with its mirror image, D-arginine, VioC performs oxidative deamination, a process that removes an amine group and introduces a ketone.
- VioC efficiently binds and transforms D-arginine, despite being designed for L-arginine.
- The reaction with D-arginine results in oxidative deamination, producing a 2-ketoacid product.
- X-ray structures reveal that the D-arginine complex maintains most of the same interactions as the native L-arginine complex, but with a different orientation.
- Isotopic labeling experiments demonstrate that the ketone oxygen in the product comes from water, not molecular oxygen.
Why is This Discovery Important?
The results of this study have far-reaching implications. Understanding how enzymes can be coaxed into performing non-native reactions opens up new possibilities for biocatalysis—using enzymes to carry out industrial chemical processes. This could lead to more sustainable and environmentally friendly ways of producing pharmaceuticals, biofuels, and other valuable compounds. It also could potentially inspire the design of new drugs that target disease-related enzymes with enhanced precision.
