Decoding the Body's Defense: How a Tiny Enzyme Could Revolutionize Medicine
"Scientists Unravel the Structure of a Key Enzyme, Paving the Way for New Treatments Against Poisoning, Addiction, and More"
In the relentless pursuit of medical breakthroughs, scientists are constantly seeking ways to understand the human body's complex mechanisms. A recent study, published in the prestigious journal PNAS, has unveiled a significant piece of this puzzle: the detailed structure of an enzyme known as butyrylcholinesterase, or BChE. This enzyme, found naturally in human plasma, holds the potential to revolutionize treatments for a variety of conditions, including poisoning and addiction.
The research, conducted using advanced cryo-electron microscopy (cryo-EM), provides an unprecedented look at how BChE functions at a molecular level. This level of detail is crucial because it allows scientists to understand how the enzyme interacts with other molecules, paving the way for the development of more effective therapies. The findings have implications for a range of health challenges, offering hope for those affected by exposure to nerve agents, substance use disorders, and other serious health concerns.
This article delves into the significance of this research, exploring the structure of BChE, its potential applications, and what these findings mean for the future of medicine. We'll examine how understanding the inner workings of this enzyme could lead to the creation of new drugs and therapies, offering renewed hope for improved health outcomes.
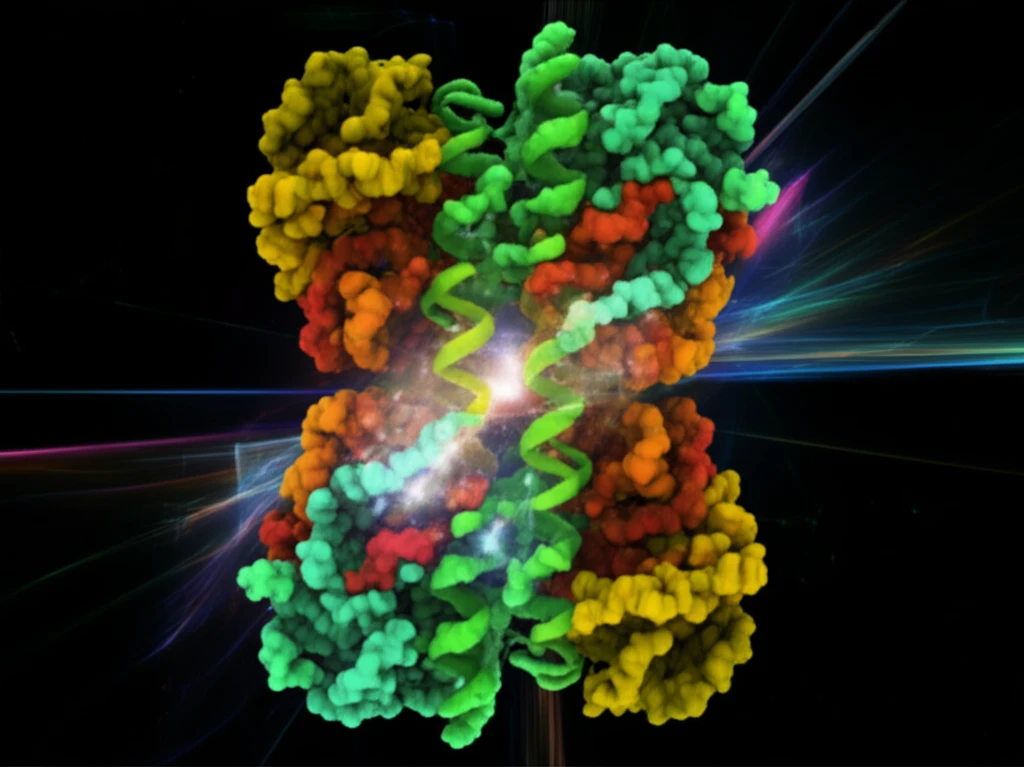
Unveiling the Structure: A Dimer of Dimers

The study's primary achievement lies in its detailed structural analysis of BChE. Researchers used cryo-EM to visualize the enzyme's structure at an incredibly high resolution. The results revealed that the BChE tetramer is organized as a "staggered dimer of dimers." This means the enzyme isn't a simple collection of four identical parts; instead, it's a more complex assembly where two pairs of enzyme molecules come together in a specific arrangement.
- Superhelical Assembly: The BChE tetramer is held together by a superhelical structure, a unique arrangement of protein components.
- Dimer of Dimers: The enzyme is composed of dimers, with each dimer also interacting with another, forming a "dimer of dimers" structure.
- WAT Helices: Key structural elements called WAT helices play a critical role in holding the enzyme together.
- High-Resolution Cryo-EM: The study used advanced cryo-EM to visualize the enzyme's structure in unprecedented detail.
A New Era for Therapeutics
The research into BChE represents a significant step forward in our understanding of human biology and its potential applications in medicine. By revealing the intricate structure of this enzyme, scientists have opened the door to developing new treatments for a range of conditions. From combating the effects of nerve agents to helping people overcome addiction, the possibilities are vast. As research continues, the detailed insights into BChE's structure will undoubtedly pave the way for a healthier future.
